Dermadry
- Projects
- Dermadry

Dermadry: an iontophoresis medical device for at-home treatment of excessive sweating
Client
Dermadry
Industry
Medical
Expertise
Hyperhidrosis, a condition that makes daily life uncomfortable
Dermadry’s founder had hyperhidrosis, a condition that causes excessive sweating. He had the idea to develop his solution using iontophoresis, a non-invasive, reliable, safe, and effective technique that uses tap water and electric current to block sweat at the most common locations affected by the condition.
Dermadry asked CLEIO to redesign the interface of its product in order to obtain the certification for its commercialization.
How CLEIO transformed the medical device for the consumer market
We proposed a new design to ensure a safe and painless use
- A highly intuitive and user-friendly interface that is easy to use at home
- A safe device that integrates many parameters to guarantee treatment effectiveness and user security
- Maximum comfort since the technology directs a small electric current through the user’s skin
- A sleek design that appeals to the consumer
A collaborative development process to innovate
We designed an affordable product thanks to a cost-effective manufacturing
The added value of the device is that it can be used at home. For this reason, the price had to be as low as possible. Special attention was given to the manufacturing cost of the final design to make the Dermadry’s device affordable to consumers.
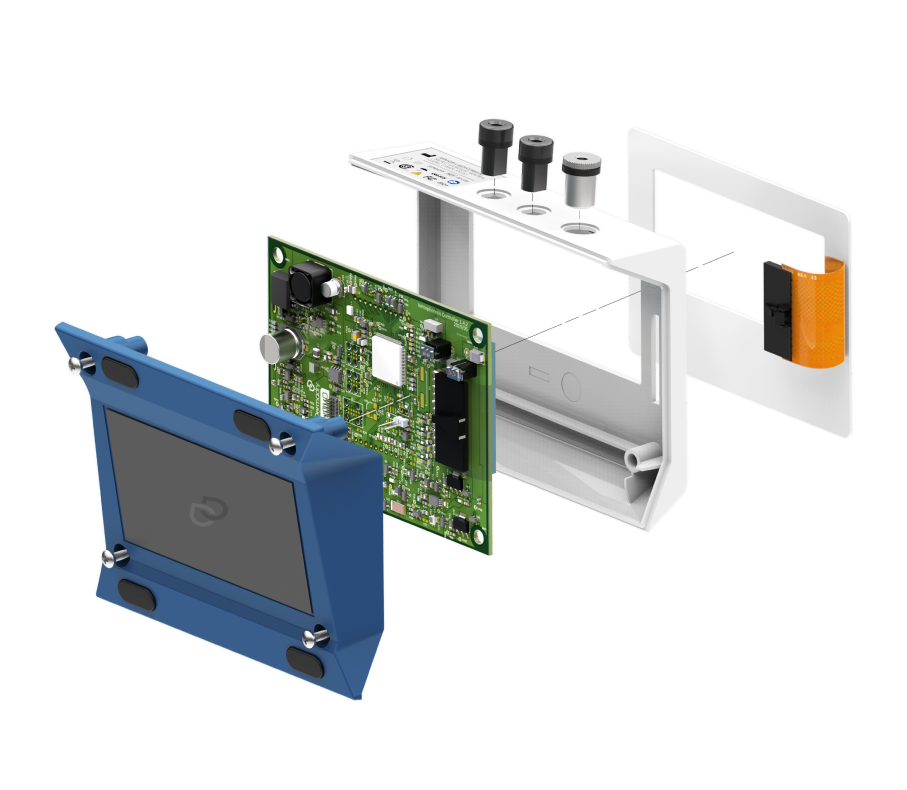
A prototype ready in 5 months and certified on the first trial
Within five months, we developed, tested, and evaluated a working prototype certified on the first trial. Today, CLEIO remains Dermadry’s primary partner. We continue to support the company in the commercialization of its products.
With the electronic parts shortage caused by the pandemic, we helped their team to find components and approve replacement parts to continue producing and meeting demand.
A medical device sold worldwide
Dermadry is the first iontophoresis device authorized by Health Canada for hyperhidrosis treatment. Marketed worldwide, it has become the reference solution for dermatologists against excessive sweating and relieves the daily life of thousands of people.
This success has allowed Dermadry to grow. Today, the company has more than close to employees.
«In addition to their expertise, CLEIO provided us with excellent communication throughout the entire process. They also made the most of every minute of our strict timeline. Working with CLEIO has ensured a solid foundation for future product development.»


